Youll find more specific groups like transition metals rare earths alkali metals alkaline earth halogens and noble gasses. Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number.
Rows on the periodic table are referred to as periods while the columns on the periodic table are referred to as groups.
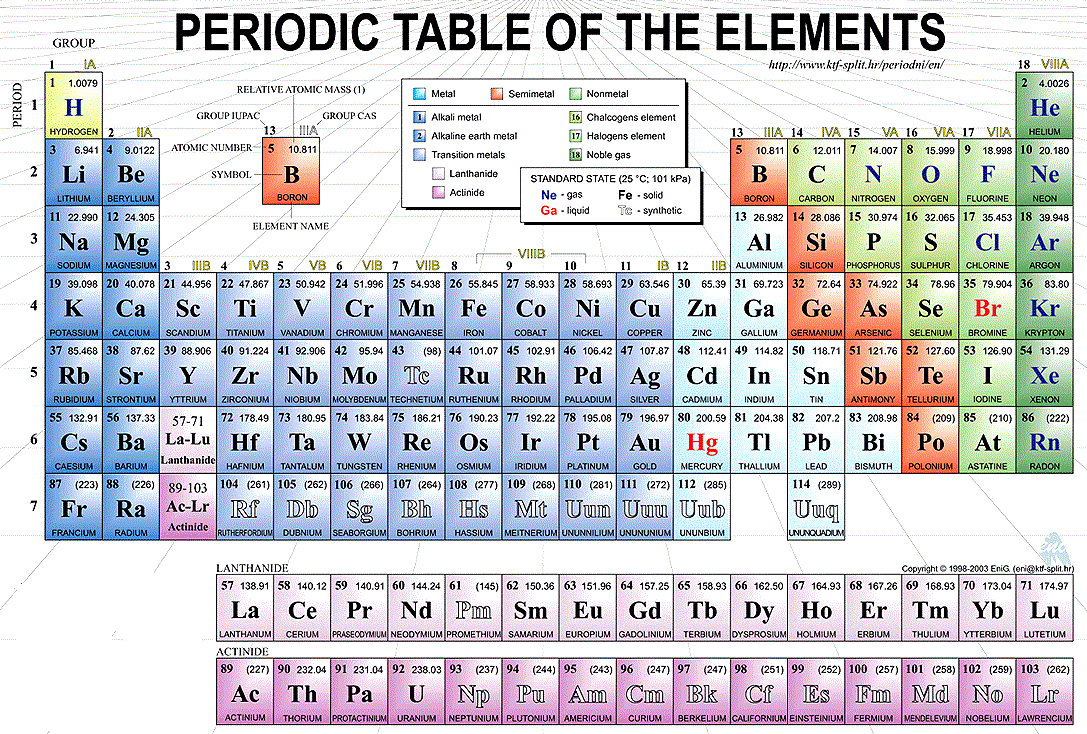
Periodic table with periods and groups labeled. Thats great to hear. The vertical columns of elements are called groups or families. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms ie the same core charge because most chemical properties are dominated by the orbital location of the outermost electron.
Click on the periodic table then click on sulfur. Poor electrical and thermal conductors. Find out in this video from the Properti.
In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elementsThere are 18 numbered groups in the periodic table. Masuzi October 24 2018 Uncategorized Leave a comment 12 Views. Periodic Table Labeled Groups In the late nineteenth century Russian scientific expert Dmitri Mendeleev distributed his first endeavor at gathering concoction elements as per their atomic loads.
Periods are horizontal rows across the periodic table while groups are vertical columns down the table. A new period begins when a new principal energy level begins filling with electrons. In general the periodicity of the periodic table in terms of periodic table blocks is clearly due.
Masuzi October 24 2018 Uncategorized Leave a comment 10 Views. All the different elements are arranged in a chart called the periodic table. Groups families periods and valence 3 1 periodic table mun ib reading the periodic table how to identify groups periods and.
Interactive periodic table showing names electrons and oxidation states. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms ie the same core charge because. The horizontal rows of the periodic table are referred as periods while the vertical columns are referred as groups.
Period 1 has only two elements hydrogen and helium while periods 2 and 3 have 8 elements. Cir Room 9 Groups Families Periods And Valence Of The Periodic Table. Atomic Structure Web Quest Name_____ _ Period____ __ Go to 1.
Periodic Table Groups And Periods Labeled. There were just around 60 elements known at that point however Mendeleev understood that when the elements were composed by weight particular sorts of elements happened in normal spans or periods. The form of the periodic table is closely related to the electron configuration of the atoms of the elements.
Visualize trends 3D orbitals isotopes and mix compounds. Atomic number increases as you move down a group or across a period. Periods 4 and 5 have 18 elements.
Whats the difference between periods and groups in the Periodic Table and why are the elements structured this way. Groups and Periods are the columns and rows of the periodic table. Here are the main features of the table.
There are multiple ways of grouping the elements but they are commonly divided into metals semimetals metalloids and nonmetals. There are seven periods in the periodic table with each one beginning at the far left. In this section you will explore how the periodic table was put together and the two main arrangement of the periodic table focusing on the groups and periods and metals and non metals.
Updated August 10 2019 Groups and periods are two ways of categorizing elements in the periodic table. The f-block columns between groups 2 and 3 are not numbered. In the periodic table of elements there are seven horizontal rows of elements called periods.
Inner Transition Metal Simple English Wikipedia The Free. Interactive periodic table with element scarcity sri discovery dates melting and boiling points group block and period information. 29 Printable Periodic Tables Free Download ᐅ Template Lab.
Periods and groups are two important characteristics of the periodic table. Modern Periodic Table Periods And Groups Chemistry For Non Majors. An elements period number represents the highest energy level that an electron in that element possesses.
With chlorine with the help of electronic structure. The horizontal rows are called periods the vertical columns are called groups. A table in which all the known.
New Periodic Table Of Elements Labeled Groups. Scroll down to the picture of the sulfur atom and its rings. Explain what the rings show and describe sulfurs valence electrons in the picture.
Periods in the periodic table. 6 Labeled Periodic Table Of Elements With Groups Of Groups. Groups and periods are two most important methods of classifying the elements in the periodic table.
The most common way the periodic table is classified by metals nonmetals and metalloids. This is what is meant by periodicity or periodic table trends. For example all the elements of group 2 have an electron configuration of E ns 2 where E is an inert gas configuration and have notable similarities in their chemical properties.
Copy the picture below 2.
Gases oxygen and solids carbon. Also include the location of the elements on the periodic table.
Groups And Periods Of The Periodic Table Metals Nonmetals And Metalloids Elements And The Periodic Table
3 DIFFERENT TYPES OF ELEMENTS.
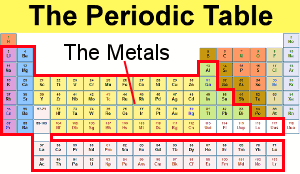
Periodic table of metals nonmetals and metalloids. Metals are placed on the left side of the periodic table Non-metals are placed on the right side of the periodic table and Metalloids are placed in the middle of the periodic table. An Introduction to General Organic and Biological Chemistry 12th. Metals are elements having the highest degree of metallic behavior.
However metalloids have a shiny and dull appearance. The only liquid non-metal is bromine. Metals Nonmetals and Metalloids Using the periodic table you can classify the elements in many ways.
Some the elements that are generally considered metalloids include. The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine. Metals Metalloids and Nonmetals Essay Compare and contrast the three different types of elements using their physical properties and explain how you are able to determine if an element is a metal nonmetal or metalloid.
Boron arsenic antimony silicon germanium selenium polonium and tellurium. The Periodic Table contains a lot of useful information on the elements. They are usually solids or gases at room temperature with low melting and boiling points boron and carbon are exceptions.
SOLIDS LIQUIDS or GASSES What makes up most of the periodic table What is the classification of these characteristics. Most of the non-metals exist in two of the three states of matter at room temperature. These have no metallic luster and do.
The periodic table of metals and nonmetals can be broken down to give you a sense of each elements characteristics. Properties of Metals Nonmetals Metalloids Created Date. Position in the Periodic Table.
Some periodic tables include a dividing line between metals and nonmetals and the metalloids may be found close to this line. One useful way is by metals nonmetals and metalloids. They are solids under standard conditions and can easily form alloys with Metals.
They form a separating boundary between the metals and nonmetals. And metalloids are the borderline. Metals are found in the left side of the periodic table.
Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals. Where are metals located on the periodic table Most metals are. Most non-metals are brittle and are neither malleable nor ductile.
Your essay must have an introduction 1 paragraph a body 3 paragraphs and a conclusion 1 paragraph. Also we can say that metalloids are present in the diagonal region of the p block on Periodic table. They are poor conductors of heat and electricity.
Nonmetals are present on the right hand side of the periodic table. Metalloids Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals. The periodic table is organized in families and periods.
Non-metals are very brittle and cannot be rolled into wires or pounded into sheets. 9182015 24543 PM. Physical Properties of nonmetals.
Periodic Table of the Elements Mg meta loids. The orange color on the Periodic table represents metalloids. On a standard periodic table all eleven elements are in a diagonal region of the p-block extending from boron at the upper left to astatine at lower right.
Metalloids are located to the right of metals and to the left of nonmetals in the periodic table. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases. METALS NONMETALS METALLOIDS Classifying elements on the Periodic Table.
Metals Nonmetals and Metalloids on the Periodic Table - YouTube A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the. The metalloids separate the metals and nonmetals on a periodic table. These elements are called metalloids.
Metals are grouped on the left side of the periodic table with an exception of a hydrogen atom. Metals are located in s p d and f blocks in the periodic table though non-metals is located in s and p blocks and metalloids are located in p block of the periodic table. In other words metalloids semimetals are located on the right side of the post transition metals and on the left side of nonmetals see above image.
Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals. Metals have a shiny appearance non-metals have a dull appearance. Difference Between Metals Nonmetals and Metalloids Definition.
Block in the. Non-metals are grouped on the right side of the periodic table. SHINY BRITTLE SEMICONDUCTOR SOLID.
Metals Nonmetals and Metalloids Chemistry 101 Periodic Table properties. The noble gases are almost completely inert. The line begins at boron B and extends down to polonium Po.
Also many periodic tables have a stair-step line on the table identifying the element groups. Development of the Periodic table Effective Nuclear Charge Atomic and Ionic sizes.
Most of them have low-density. Metals shiny solids are room temperature except mercury which is a shiny liquid element with characteristic high melting points and densities.
Definition Of Metals Chemistry Dictionary
The elements in group 1 are called the alkali metals.
Characteristics of metals on the periodic table. List and explain the properties of metals Remember that metals are on the left and bottom of the periodic table. Metals prefer to eliminate electrons in their last shell. Good conductors of heat.
Some elements have properties that are not typical. Most elements are metalsThey are usually shiny very dense and only melt at high temperatures. The highest density alkali metal is Cesium Cs element.
The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine. The lowest density of alkali metal is lithium Li element. Transition metals Because this group contains so many elements they exhibit a wide range of properties.
Group 1 - physical properties Group 1 contains elements placed in a vertical column on the far left of the periodic table. Solid at room temperature with the exception of mercury usually shiny high melting point good conductor of heat good conductor of electricity low ionization energies low electronegativities malleable able to be pounded into sheets ductile. The periodic table on the left separates elements into three groups.
The periodic table of metals and nonmetals can be broken down to give you a sense of each elements characteristics. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases. They are malleable.
2 3 In actual practice the f-block lanthanide and actinide series are also considered transition metals and are called inner transition metals. Also many periodic tables have a stair-step line on the table identifying the element groups. The metals green in the table nonmetals orange and metalloids blue.
Most elements are metals. On the periodic table metals are separated from nonmetals by a zig-zag line stepping through carbon phosphorus selenium iodine and radon. Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals.
Common characteristics of metals. Based on this group. Group 1 is on the.
S block p block d block f block. Metals Characteristics Variation in Periodic Table Metals in periodic table. The block of transitional metals which is present in middle part of the modern periodic table includes elements of group IB to VIIIB ie.
Thus metals are electropositive elements with relatively low ionization energies. The elements here are beryllium magnesium calcium strontium barium and radium. Based on the periodic trends in the last 3 sections this means that they are usually bigger more likely to lose electrons and less likely to gain electrons than the non-metals.
The Periodic Table contains a lot of useful information on the elements. The metals share several common properties including. Many of the properties of metals including a large atomic radius low ionization energy and low electronegativity are because the electrons in the valence shell of metal atoms can be removed easily.
Their shape can be easily changed into thin wires or sheets without breaking. Poor conductors of heat. Therefore they form cations.
These elements and those to the right of them are nonmetals. These metals have properties that you normally associate with the metals you encounter in everyday life. Alkaline earth metals are the elements present in main group 2 2A of the periodic table.
The noble gases are almost completely inert. Most often they are good conductors of heat and electricity. Lithium Li Sodium Na Potassium K because their densities are smaller than the density of water 1 gmcm³.
Many scientists describe a transition metal as any element in the d-block of the periodic table which includes groups 3 to 12 on the periodic table. This includes the alkali metals alkaline earth metals transition metals lanthanides and actinides. With the exception of hydrogen all elements that form positive ions by losing electrons during chemical reactions are called metals.
For example excluding hydrogen all of the elements in Group 1 on the very left-hand side of the periodic table are called alkali metals. They are characterized by bright luster hardness ability to resonate sound and are excellent conductors of heat and electricity. The line begins at boron B and extends down to polonium Po.
Mercury a metal has. Metals are located in all blocks in periodic table. Alkali elements that float on the water surface.
They are ductile they can be drawn into thin wires. The metalloids separate the metals and nonmetals on a periodic table. They are more dense and melt faster than metals in groups 1 and 2.
They are solid with the exception of mercury Hg a liquid. 3 to 12 these elements have last electron in d-subshell their properties are midway between those of s-block p-block so they are called Transitional elements. They are shiny good conductors of electricity and heat.
Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals. Alkali metals are soft and silvery and react violently.
Oxygen nitrogen sulfur while most metals form cations eg. Most elements are metals.
Classification Of Elements In The Periodic Table Color Coding Yellow Download Scientific Diagram
Alkali metals and alkaline earth metals always form cations.

What elements are metals on the periodic table. The six alkaline earth metals are. Metals form ionic bonds with non-metals. These metals are considered to be both very rare and of high value.
Metals make up more than 70 percent of the Periodic Table of the Elements but many metals are unfamiliar to most of us. Remember thats the easy list. Eric Scerri 2007 The periodic table.
Alkaline Earth Metals The alkaline earth metals are found in column 2 on the left side of the Periodic Table. Most elements can be considered metals. They can be described as a lattice of positive ions surrounded by a cloud of delocalized electrons.
Subsequently one may also ask what percent of the elements on the periodic table are found in nature. The p-block elements together with the s-block elements the tall groups on the Periodic Table Electronegativity A measure of the ability of an atom in a chemical compound to attract electrons. Of those the two most rare metals are rhodium Rh and osmium Os.
This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides. This list contains the 118 elements of chemistry. Chemical elements alphabetically listed The elements of the periodic table sorted by name in an alphabetical list.
Similarly one may ask what are the cations in the periodic table. Science Picture CoGetty Images. The reactivity increases as you move down the periodic table so cesium reacts explosively.
Periodic Table Metals A metal is an element that readily loses electrons to form positive ions cations and has metallic bonds between metal atoms. All of the elements in the alkali metal group are extremely reactive. Most elements can be considered metals.
Chemistry Metals and the Periodic Table of the Elements We use metals everyday. Lucky for you the periodic table is excellent at organizing elements and you will find each of these groups in specific areas of the periodic table. Most other nonmetals typically form anions eg.
Youll find metals like iron nickel chromium aluminum and cobalt in objects such as cookware cars and skyscrapers. The periodic table is organized in families and periods. Using the periodic table you can classify the elements in many ways.
If you put pure sodium or potassium metal in water the result will be a fire. This is a list of the 118 chemical elements which have been identified as of 2021. They are grouped together in the middle to the left-hand side of the periodic table.
Heres a quick list. A popular visualization of all 118 elements is the periodic table of the elements a convenient tabular arrangement of the elements by. Discovery of the Elements The Movie YouTube 118 The History Of Metals Timeline.
On the periodic table there is a family of eight elements known as the precious metals including elements 44 47 like silver and 76 79 like gold. Although separate on the periodic table lanthanides and actinides are really specific types of transition metals. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides.
The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties. Metals In The Periodic Table So because most elements of the Table are metals it makes sense to begin by looking at them. Its story and its significance Oxford.
Actinide Metals Lanthanide Metals Alkali Metals Alkaline-Earth Metals Rare Metals Rare-Earth Metals and Transition Metals. One useful way is by metals nonmetals and metalloids. Click on any elements name for further chemical properties environmental data or health effects.
A timeline showing the discovery of metals and the development of metallurgy. They are generally harder and denser than alkali metals have 2 electrons in their outermost s sub-shell and each make a distinct color in their flames. Halogens always form anions.
They are grouped together in the middle to the left-hand side of the periodic table. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. The pure element would like explode on contact with skin.
Metals reside on the left side of the table while non-metals reside on the right. History of Elements of the Periodic Table. Timeline of Element Discoveries.
Metals In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to. A chemical element often simply called an element is a species of atoms which all have the same number of protons in their atomic nuclei ie the same atomic number or Z. The metals are one of the three groups of elements as.
If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po.
A section of the periodic table showing metals and non-metals The main groups are numbered from 1 to 7 going from left to right and the last group on the right is group 0. Groups and periods are two most important methods of classifying the elements in the periodic table.
Cir Room 9 Groups Families Periods And Valence Of The Periodic Table
Atomic number increases as you move down a group or across a period.
Periodic table with groups and periods labeled. Period 1 has only two elements hydrogen and helium while periods 2 and 3 have 8 elements. New Periodic Table Of Elements Labeled Groups. Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number.
In this section you will explore how the periodic table was put together and the two main arrangement of the periodic table focusing on the groups and periods and metals and non metals. With chlorine with the help of electronic structure. There are seven periods in the periodic table with each one beginning at the far left.
A new period begins when a new principal energy level begins filling with electrons. The atomic number is the number of protons and neutrons present in the cores of a component. 29 Printable Periodic Tables Free Download ᐅ Template Lab.
The elements are orchestrated from left to directly arranged by their expanding atomic number. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms ie the same core charge because. Periodic Table Groups And Periods Labeled.
This table organizes the elements by increasing atomic number into rows periods in which the columns groups share recurring periodic physical and chemical properties. Explain what the rings show and describe sulfurs valence electrons in the picture. The most common way the periodic table is classified by metals nonmetals and metalloids.
All the different elements. The section in the. An elements period number represents the highest energy level that an electron in that element possesses.
Groups families periods and valence 3 1 periodic table mun ib reading the periodic table how to identify groups periods and. This interactive periodic table of element groups arranges the chemical elements according to periodicity or common properties. Copy the picture below 2.
Masuzi October 24 2018 Uncategorized Leave a comment 10 Views. The vertical columns of elements are called groups or families. Poor electrical and thermal conductors.
Periods are horizontal rows across the periodic table while groups are vertical columns down the table. Modern Periodic Table Periods And Groups Chemistry For Non Majors. Family Definition in Chemistry.
The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms ie the same core charge because most chemical properties are dominated by the orbital location of the outermost electron. Thats great to hear. The f-block columns between groups 2 and 3 are not numbered.
Are arranged in a chart called the periodic table. The Periodic Table is the table that masterminds the concoction elements in an efficient structure that is in a plain structure. Noble gases are all colorless.
The horizontal rows are called periods. Periodic Table Elements Groups Periods Priyamstudycentre. Inner Transition Metal Simple English Wikipedia The Free.
Interpreting The Periodic Table. Periods and groups are two important characteristics of the periodic table. Families and Periods In the periodic table of elements there are seven horizontal rows of elements called periods.
Rows on the periodic table are referred to as periods while the columns on the periodic table are referred to as groups. 6 Labeled Periodic Table Of Elements With Groups Of Groups. A table in which all the known.
The elements in the last group on the periodic table Group 18 are called the noble gases. Noble gases are all colorless odorless and extremely un-reactive. Groups and Periods are the columns and rows of the periodic table.
Here are the main features of the table. Click on the periodic table then click on sulfur. The horizontal rows of the periodic table are referred as periods while the vertical columns are referred as groups.
Masuzi October 24 2018 Uncategorized Leave a comment 12 Views. Interactive periodic table with element scarcity sri discovery dates melting and boiling points group block and period information. Atomic Structure Web Quest Name_____ _ Period____ __ Go to 1.
Periods 4 and 5 have 18 elements. Scroll down to the picture of the sulfur atom and its rings. The Difference Between an Element Group and Period.
The vertical columns are called groups. The periodic table summarizes various properties of the elements allowing chemists to derive relationships between them and to make predictions about compounds and potential new ones. Updated August 10 2019 Groups and periods are two ways of categorizing elements in the periodic table.
In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elementsThere are 18 numbered groups in the periodic table. The Periodic Properties of the Elements.