For a complete list of Beginners posts see Financial Crisis for Beginners. These products may take traditional securities such as an.

Structured Products Chartered Opus
Structured products contain within them a promise thats common to any hedge fund namely the potential for an absolute return in all markets and market conditions.

Structured products for dummies. Welcome to the world of structured products. Investec Structured Products is a trading name of Investec Bank plc. Structured notes arent direct investments but derivatives.
A structured product also known as a market-linked investment is a pre-packaged investment strategy based on a single security a basket of securities options indices commodities debt issuance or foreign currencies. Before you even start thinking about using structured investments you need to understand exactly what youre getting. Structured data tells them its a product image.
Structured Investment Categories M organ Stanley Structured Invest - ment products can be divided into five basic categories each offering structural characteristics designed to help investors realize specific financial objectives. Much maligned structured products are back on the investors radar as we seek post-retirement solutions that pay a sustainable income. In 2012 I had just negotiated a severance package after 11 years at my former employer.
What Are Structured Notes. It is a member of the London Stock Exchange registered under Financial Services Register reference 172330. Structured Products can be loosely defined as a savings or investment products where the return is linked to an underlying asset with pre-defined features maturity date coupon date capital protection level.
Creditor risk is the primary concernShould the issuing bank go out of business the note could be worthless as with any other corporate bond regardless of whether the underlying investments have a positive return or not. There is no single structure in Structured Products. The five major structured investment strategies are.
Closely examining any investment product available in the UK should alert you to the risks involved some of the structured products sold on the market can be very complicated and potentially toxic even for a sophisticated investor. Structured products are a class of investment created to offer a return that differs from the returns available directly from the underlying investment. Structured products are created by investment banks and often combine two or more assets and sometimes multiple asset classes to create a product that pays out based on the performance of those.
Youll learn which product types make sense in which market phases how to trade structured products and what the associated risks are. Structured Products are pre-packaged investments strategies. Some are based on options commodities currencies a basket of securities among many others.
Well introduce you to products that can help you to earn attractive returns regardless of whether the markets are going up down or simply marching in place. A structured note is a debt product whose return is linked to the performance of one or more underlying assets or benchmarks. Investec Bank plc is authorised by the Prudential Regulation Authority and regulated by the Financial Conduct Authority and the Prudential Regulation Authority.
Structured notes risks include liquidity pricing and credit risk of the issuer among other things. These market-linked investments are called Structured Products. It may be the interest that is payable on the structured note andor the principal repayment that is linked to the performance of the asset or benchmark.
N Market-Linked Notes and Market-Linked Deposits. This book is highly recommended for investors and asset managers but is also be useful for salespersons junior traders or as a textbook for teachers and students. Read the book How to Invest in Structured Products and optimize your chances of success.
They belong to the range of products with non-traditional investment strategies. Making a profit when shares rise in. The humble structured product otherwise known in the UK as a structured investment the two terms are used interchangeably is introduced here.
Most structured products are made up of a combination of cash and derivatives. They track the value of another product. They dont necessarily take the risk out of investing but are designed to help you understand the likely outcomes of your investment.
This is more of an advanced beginners topic I already covered CDOs collateralized debt obligations in my first Beginners article but I imagine that most of our readers are already familiar with structured products. A Structured Product SP is a hybrid investment instrument which is used to improve the return on a fixed income or an equity instrument while reducing the risk on the product using a derivative instrument as an insurance on the downsideThe layer of derivatives gives it the flexibility needed to blend with a portfolio and enhance its risk. Structured Products are a mix of traditional financial instruments stocks and bonds with one or more elements that utilize derivatives.
But should they be avo. What exactly is a Structured Product. At least many people know that first a bunch of securities are pooled together and then they.
Structured notes combine bonds and additional investments to offer the features of both debt assets and investment assets. A structured note is a hybrid security It combines the features of multiple different financial products into one. Structured products are pre-packaged investments that normally include assets linked to interest plus one or more derivatives.
An image is labeled as a product image specific words are labeled as a product review etc. Rules Are Necessary For Organizing Information.
The periodic table presents all the elements known so far which are organized and located according to their characteristics and relationship between them in group periods blocks and metals metalloids and non-metals. 1 This group of elements is made up of all different states of matter solid liquid and gas a Nonmetals b Metals c Metalloids 2 These elements always have a shiny luster a Nonmetals b Metals c Metalloids 3 These elements are located in a stair step pattern on the periodic table a Nonmetals b Metals c Metalloids 4 Elements in this group are good conductors of heat AND electricity a Nonmetals b Metals c Metalloids 5 This tells you.

Printable Periodic Tables Science Notes And Projects Science Notes Periodic Table Periodic Table Of The Elements
One useful way is by metals nonmetals and metalloids.
Periodic table groups metals nonmetals metalloids. Most elements are metals. High density High strength Resistance to corrosion The SHOULD be up in the regular table but they simply would not fit without messing up the make-up of the table as we have it. The IUPAC definition defines a transition metal as an element whose atom has a partially filled d sub-shell or which can give rise to cations with an incomplete d sub-shell.
These elements are called metalloids. Metalloids Some elements between the metals and non-metals in the periodic table have properties which are a mixture of the properties of metals and non-metals. Metals left side of a period generally have a lower electron affinity than nonmetals right side of a period with the exception of the noble gases.
Periodic table groups metals nonmetals metalloids periodic table groups transition metals periodic. Metals Nonmetals Metalloids The periodic table on the left separates elements into three groups. The periodic table is made up of 18 groups of elements organized in vertical columns numbered from 1 to 18 from left.
Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals. Periodic Table Of The Elements Metals. Best Of Periodic Table Groups Metals Metalloids And Nonmetals.
It is designed to be used in conjunction with a notes packet for. The metalloids also known as semi-metals are placed between metals and non-metals in the periodic table of elements. Search Help in Finding Periodic Table Metals NonMetals and Metalloids - Online Quiz Version Periodic Table Metals NonMetals and Metalloids online quiz.
The Position Of The Inner Transition Elements Is Toppr Com. This is an online quiz called Periodic Table Metals NonMetals and Metalloids There is a printable worksheet available for download here so you can take the quiz with pen and paper. Metals Nonmetals Metalloids - Maze chase.
The noble gases are almost completely inert. The periodic table is organized in families and periods. The metals green in the table nonmetals orange and metalloids blue.
The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine. Also many periodic tables have a stair-step line on the table identifying the element groups. In chemistry the term transition metal or transition element has three possible definitions.
They are found in a stair step line that helps differentiate metals from non-metals in this element table. Metals Nonmetals and Metalloids Using the periodic table you can classify the elements in many ways. According to their shared physical and chemical properties the elements can be classified into the major categories of metals metalloids and nonmetals.
This is a brief overview of how the periodic table is divided into three major groups called Metals Non-metals and Metalloids. Shiny Ductile Good Conductor Poor insulators Located on the Left side of the periodic table Mallable Metalloids. Metalloids-Metalloids are the group of certain elements which has both the.
Examples of non-metals are carbon nitrogen etc. Non-Metals are present on the right side of the periodic table. Metallic properties decrease as we move from left to right across the groups.
They are usually shiny very dense and only melt at high temperatures. Shiny or dull Brittle or malleable Semi-Conductors Staircase on the Periodic Table Non-metals. Look at Image Dull Brittle Poor Conductors Good Insulators Located on the right side Not Ductile.
The metalloids separate the metals and nonmetals on a periodic table. There are seven elements that are classified as metalloids and placed in Group 13 14 15 16 and 17. Z e e s These are still metals and they share the same patterns as all of the other metals in the table.
These elements are distinctive in that they typically have low melting and boiling points dont conduct heat or electricity very well and tend to have high ionization energies and electronegativity values. Properties of these series. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases.
The line begins at boron B and extends down to polonium Po. Many scientists describe a transition metal as any element in the d-block of the periodic table which includes groups. The nonmetals or non-metals are a group of elements located on the right side of the periodic table except for hydrogen which is on the top left.
Job costing and process costing systems also have their significant differences. Job costing is more likely to be used for billings to customers since it details the exact costs consumed by projects commissioned by customers.
Process costing on the other hand is used when companies offer a more standardized product.
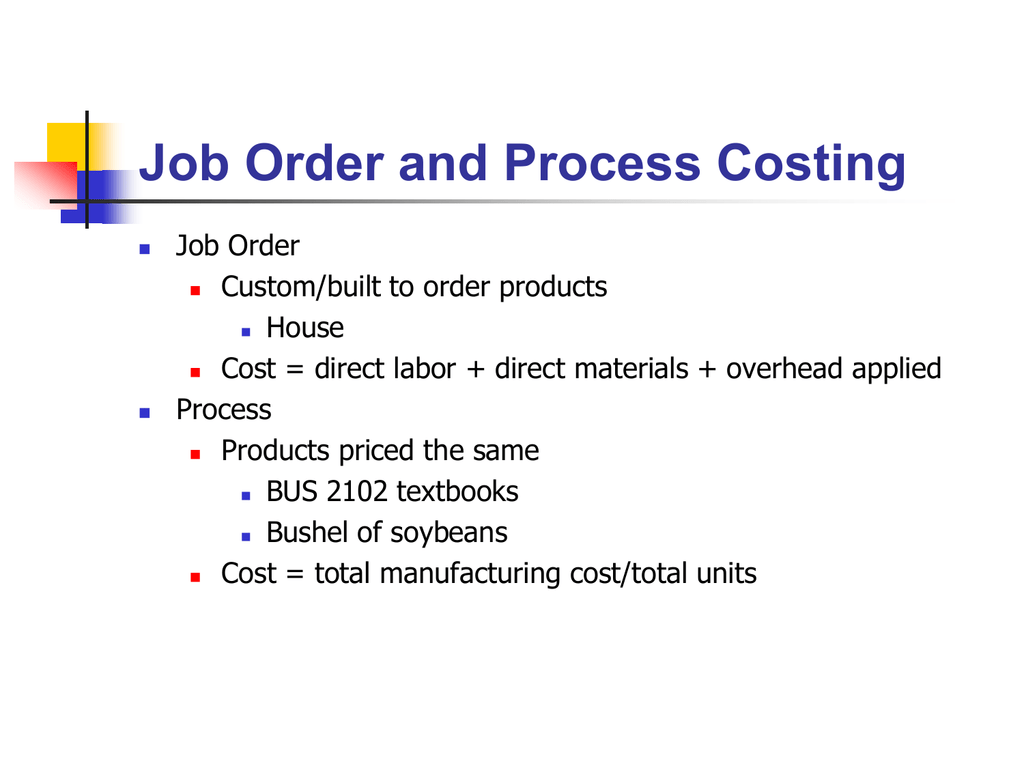
Job order costing and process costing are. Job order costing is an accounting system that traces the individual costs directly to a final job or service instead of to the production department. Process of Job Order Costing 1. That form of costing which applies where standardised goods are produced and production is in continuous flow the products being homogeneous.
The key difference between job order costing and process costing is that job costing is used when products are manufactured based on customer specific orders whereas process costing is used to allocate costs in standardized manufacturing environments. It is used when goods are made to order or when individual costs are easy to trace to individual jobs assuming that the additional information provides value. Each job must have its own identity which is called Job Code to make sure that all relevant.
Companies that use process costing produce a single product either on a continuous basis or for long periods. It is used when goods are made to order or when individual costs are easy to trace to individual jobs assuming that the additional information provides value. 81 Job Order v.
The process costing is used for the costing of more standardized products that are usually produced in large volumes. What is Job Costing. There are a few different types of process costing which can be used depending on your situation.
Process costing aggregates costs and so requires less record keeping. However in process costing the cost of each job is determined. Slide 312 Bhimani Horngren Datar and Rajan Management and Cost Accounting 5th Edition Pearson Education Limited 2012 Job-Costing and Process-Costing Systems Continued In a job-costing system the cost object is an individual unit batch or lot of a distinct product or service called a job.
Ii The job is the cost unit and costs are collected. The job order costing is used for the costing of products that are more unique and customizable. No matter who the customer is they all end up receiving the same product.
Process costing and job order costing are both acceptable methods for tracking costs and production levels. How to Calculate Process Costing. Job costing is used in cases where products produced are unique and process costing is used for the standardized products produced.
This is usually applied to small production orders. Process costing works better for things such as manufacturing aspirins. The type of costing method you use depends on the type of business youre running.
The system a company uses depends on the nature of the product the company manufactures. The job order costing is used for the costing of products that are more unique and customizable. Ultimately job order costing and process costing are cost accounting systems which are both viable the key is to identify unique circumstances and needs of your business.
Job Order Costing versus Process Costing. Every time materials withdraw from the warehouse the requested person needs to identify the job. Much more record keeping is required for job costing since time and materials must be charged to specific jobs.
Companies that use job costing work on many different jobs with different production requirements during each period. Difference between Job Costing and Process Costing. Process Costing Job Order Costing versus Process Costing.
A hand carved marble statue would get job order costing. Job Order Costing vs Process Costing As an example law firms or accounting firms use job order costing because every client is different and unique. Job order costing looks at how much an individual item costs to manufacture.
Job costing refers to calculating the cost of a special contract work order where work is performed as per clients or customers instructions. Job costing is used for very small production runs and process costing is used for large production runs. In job costing the cost is calculated after the completion of the job.
Process costing looks at the cost of making thousands or millions of individual items. A costing method in which the costs which are charged to various processes and operations is ascertained is known as Process Costing. Job order costing is an accounting system that traces the individual costs directly to a final job or service instead of to the production department.
Some companies use a single method while some companies use both which creates a hybrid costing system. Types of products produced. Job Costing Process Costing i The form of specific order costing which applies where the work is undertaken to customers special requirements.
Job costing also known as job order costing and process costing are cost accounting systems designed to help businesses keep track of all the costs they have to pay to produce a product or deliver a service. Job order costing and process costing are systems of collecting and allocating costs to units of production. This applies to products that are produced in small volumes whereas process costing is used for the costing of more standardized products that are usually produced in large volumes.
Valence electrons participate in chemical reaction and elements with the same number of valence electrons react similarly. This theory is based on the idea that valence electrons in a molecule tend to repel each other to create more space around them.

Introduction To Molecules Directions To Move Forward Click The Arrow Or Click The Button With The Correct Answer Try It Now To Move Ahead Yes Ppt Download
Therefore elements with the same number of valence electrons are grouped together in the.

Why are valence electrons important in chemical reactions. This is important in the formation of ionic compounds and covalent compounds. How valence electrons determine molecular shape. Lets use methane molecule to further explain this.
Valence electrons are important in chemical reactions because they are involved in chemical bonding and bonds go through significant changes during a. Chemical reactions may aslo absorb energy which is indicated by an _____ in temperature. Since the attraction between valence electrons and the nucleus of an atom is less valence electrons can easily be removed than the electrons in the inner orbitals.
They are important because they determine how an atom will react. Valence Electrons The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction. Valence electrons are the electrons in the outermost energy level of an atom in the energy level that is farthest away from the nucleus.
In a single covalent bond both atoms in the bond contribute one valence electron in order to form a shared pair. Why are valence elecrtons important. Since filled d or f subshells are seldom disturbed in a chemical reaction we can define valence electrons as follows.
Valence electrons also tend to be the electrons that specifically participate in a chemical bond like ionic bonds or covalent bonds. Chemical bonding often requires the exchange or sharing or valence electrons. Why are valence electrons especially important in chemical reactions.
In this type of diagram an elements chemical symbol is surrounded by dots that represent the valence electrons. Find an answer to your question Why are valence electrons important. A valence electron is an electron in the outer shell that is shared or exchanged in a chemical reaction.
For more information about valence electrons core electrons and how they related to chemical reactions please see UC Daviss chem wiki. Valence electrons are responsible for chemical reactions and chemical bonding of an atom. See full answer below.
In chemistry and physics a valence electron is an outer shell electron that is associated with an atom and that can participate in the formation of a chemical bond if the outer shell is not closed. Because valence electrons are so important atoms are often represented by simple diagrams that show only their valence electrons. The simplest way to identify the valence electrons is to look for the highest number in the electron configuration of an atom the principal quantum number.
The octet rule is a chemical rule of thumb that reflects the theory that main group elements tend to bond in such a way that each atom has eight electrons in its valence shell giving it the same electronic configuration as a noble gasThe rule is especially applicable to carbon nitrogen oxygen and the halogens but also to metals such as sodium or magnesium. To predict molecular shape we usually use a theory called the valence shell electron pair VSEPR repulsion theory. Valence electrons are the most exposed of all the electrons essentially acting as a protective barrier for the rest of the atom.
Valence electrons are important because they participate in chemical reactions and the elements classified into different groups on the basis of number of valence electrons. The number of electrons in an atoms outermost valence shell governs its bonding behavior. When chemists study chemical reactions they study the transfer or sharing of electrons.
Electrons are an essential part of an atom. Because they are in the highest energy level they are generally the most involved in chemical reactions since they are the easiest to transfer. Why Are Valence Electrons Important.
KawaiiPotato42 KawaiiPotato42 11282016 Chemistry Middle School Why are valence electrons important. By writing an electron configuration Youll be able to see how many electrons occupy the highest energy level. Another way to think of valence electrons is that they are the outermost electrons in an atom so they are the most susceptible to participation in chemical bond formation or ionization.
Nissa has a masters degree in chemistry and has taught high school science and college level chemistry. The electrons that occupy the outer most shell of an atom are called valence electrons. These are called electron dot diagrams and three are shown below.
Unlike protons and neutrons valence electrons. The electrons on an atom that are not present in the previous rare gas ignoring filled d or f subshells.